Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
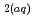 , HSiO
, HSiO
 and SiO
and SiO

Walker, C.*; Anraku, Sohtaro; Oda, Chie; Mitsui, Seiichiro; Mihara, Morihiro
no journal, ,
Equilibrium constants ( ) describing the formation reactions of SiO
) describing the formation reactions of SiO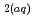 , HSiO
, HSiO
 and SiO
and SiO
 can be used to derive their thermodynamic properties. However, SiO
can be used to derive their thermodynamic properties. However, SiO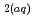 was derived from inaccurate quartz solubility data, HSiO
was derived from inaccurate quartz solubility data, HSiO
 was not extrapolated to zero ionic strength and SiO
was not extrapolated to zero ionic strength and SiO
 is routinely ignored because of its restricted dominance to very high pH
is routinely ignored because of its restricted dominance to very high pH  13 solutions. Using quartz and water data as well known "anchor" points,
13 solutions. Using quartz and water data as well known "anchor" points,  values describing the formation reactions of SiO
values describing the formation reactions of SiO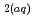 , HSiO
, HSiO
 and SiO
and SiO
 were revised to derive new thermodynamic properties and revised Helgeson-Kirkham-Flowers equation of state (r-H-K-F EoS) parameters. These properties and parameters can be used in the derivation of the thermodynamic properties of other Si bearing aqueous species/complexes and the thermodynamic properties of cementitious, clay, zeolite, and rock forming minerals, and in calculating groundwater compositions relevant to the geological disposal of radioactive wastes.
were revised to derive new thermodynamic properties and revised Helgeson-Kirkham-Flowers equation of state (r-H-K-F EoS) parameters. These properties and parameters can be used in the derivation of the thermodynamic properties of other Si bearing aqueous species/complexes and the thermodynamic properties of cementitious, clay, zeolite, and rock forming minerals, and in calculating groundwater compositions relevant to the geological disposal of radioactive wastes.
Terashima, Motoki; Beppu, Hikari*; Endo, Takashi*; Nemoto, Kazuaki*; Amano, Yuki
no journal, ,
no abstracts in English
Francisco, P. C. M.; Ishidera, Takamitsu; Tachi, Yukio
no journal, ,
Miyajima, Yusuke*; Saito, Ayaka*; Kagi, Hiroyuki*; Yokoyama, Tatsunori; Hirata, Takafumi*; Roberts, N. M. W.*; Horstwood, M.*
no journal, ,
Direct age determination of carbonate minerals provides valuable insight to paleoenvironmental change, tectonics, and sub-surface fluid-flow. The U-Pb method using laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS) is a key technique to date natural carbonates such as fault-hosted calcite that cannot be dated by biostratigraphy or Sr isotope stratigraphy. A natural calcite cement WC-1 (254.4  6.4 Ma) is suitable as a reference material to correct elemental fractionation with LA-ICP-MS. However, the resulting uncertainty on a sample date is limited by the heterogeneity of U and Pb distribution in the reference material. Our objective is to synthesize calcite with homogeneous U and Pb concentrations and isotope ratios to be used as novel reference materials. To achieve this, we incorporate incompatible U and Pb into calcite through heat-induced crystallization from amorphous calcium carbonate precipitated from U, Pb-doped reagent solution. LA-ICP-MS analyses of results so far revealed that the U/Ca and Pb/Ca ratios in the synthetic calcites were generally homogeneous with relative standard deviation (2SD, n = 10) better than 4% and 7%, respectively. The Pb/Ca homogeneity of calcites doped with
6.4 Ma) is suitable as a reference material to correct elemental fractionation with LA-ICP-MS. However, the resulting uncertainty on a sample date is limited by the heterogeneity of U and Pb distribution in the reference material. Our objective is to synthesize calcite with homogeneous U and Pb concentrations and isotope ratios to be used as novel reference materials. To achieve this, we incorporate incompatible U and Pb into calcite through heat-induced crystallization from amorphous calcium carbonate precipitated from U, Pb-doped reagent solution. LA-ICP-MS analyses of results so far revealed that the U/Ca and Pb/Ca ratios in the synthetic calcites were generally homogeneous with relative standard deviation (2SD, n = 10) better than 4% and 7%, respectively. The Pb/Ca homogeneity of calcites doped with  1
1  g/g Pb was up to ~25%. The
g/g Pb was up to ~25%. The 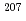 Pb/
Pb/ Pb ratio of the synthetic calcites was very homogeneous with
Pb ratio of the synthetic calcites was very homogeneous with  1% variation, whereas the
1% variation, whereas the  U/
U/ Pb ratio was less homogeneous (2%
Pb ratio was less homogeneous (2% 7% variation). To evaluate the suitability of the synthetic calcite as a reference material, we measured the age of WC-1 using the synthetic calcite for elemental fractionation correction; we could date WC-1 with a precision and accuracy of ~3% (246.6
7% variation). To evaluate the suitability of the synthetic calcite as a reference material, we measured the age of WC-1 using the synthetic calcite for elemental fractionation correction; we could date WC-1 with a precision and accuracy of ~3% (246.6  7.3 Ma). This method is a promising alternative in addition to characterising natural carbonate materials for elemental and isotopic composition.
7.3 Ma). This method is a promising alternative in addition to characterising natural carbonate materials for elemental and isotopic composition.
Tokunaga, Kohei; Kozai, Naofumi; Takahashi, Yoshio*
no journal, ,
no abstracts in English
Takahashi, Yoshio*; Tsuboi, Hiroyuki*; Yamaguchi, Akiko
no journal, ,
no abstracts in English